How do you write the orbital diagram for phosphate? Socratic
C We obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore the [Ne] closed shell. This gives a valence-electron configuration of 3 s2 3 p3. Exercise 6.8.1 6.8. 1. Draw an orbital diagram and use it to derive the electron configuration of chlorine, Z = 17.
34 Build The Orbital Diagram For The Ion Most Likely Formed By
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).

Diagram representation element phosphorus Vector Image
Phosphorus electron configuration. Electronic configurations of elements. P (Phosphorus) is an element with position number in the periodic table. Located in the : 44 ℃. Silicon (Si) electron configuration Sulfur (S) electron configuration. Electronic configuration of the Phosphorus atom. Valence electrons. Orbital diagram.
Phosphorus Electron Configuration (P) with Orbital Diagram
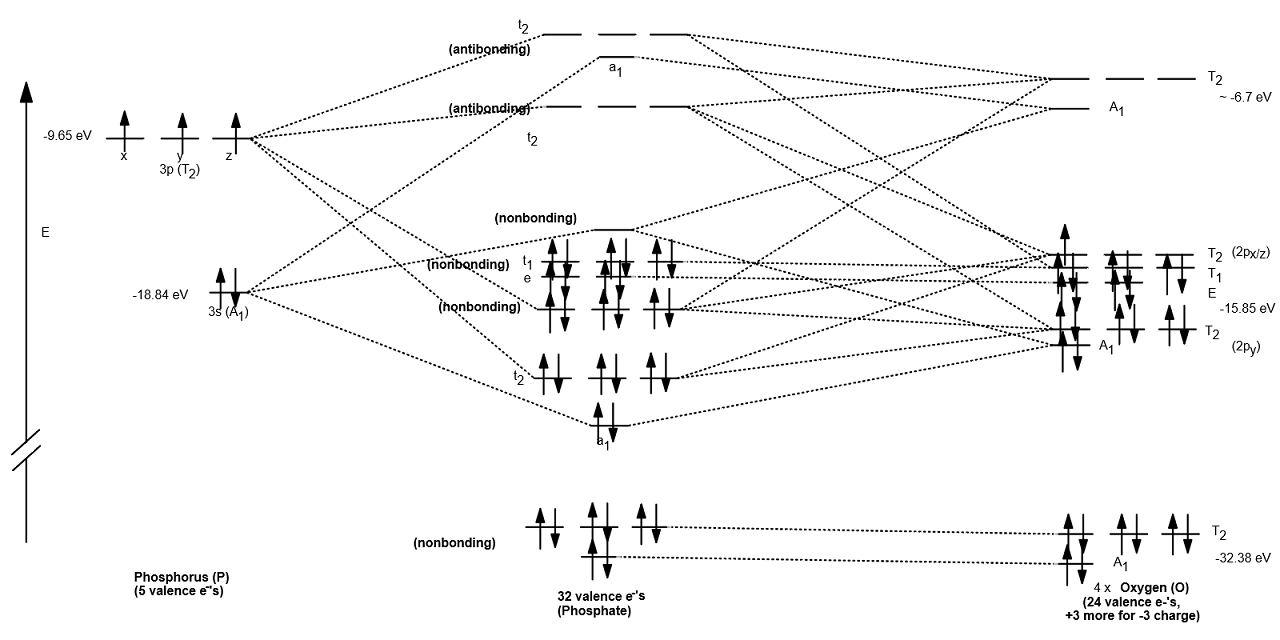
Phosphate has all electrons paired. The nonbonding orbitals are nonbonding either due to mismatched symmetries, overly different inner-atom/outer-atom orbital energies, or interactions between both high-lying and low-lying orbitals. There are 22 nonbonding electrons, which accounts for all 11 lone pairs of electrons that belong to the oxygen atoms.

Aufbau Diagram For Phosphorus
Study the phosphorus electron configuration with an orbital diagram and develop a decent understanding of this chemical element for yourself. We are going to dedicate this article to all our readers who are willing to explore Phosphorus with the utmost clarity.All such individuals whether they are into their academics or the general stream of life can make the most of this article for their.
Solved Which orbital diagram corresponds to phosphorus (P)?
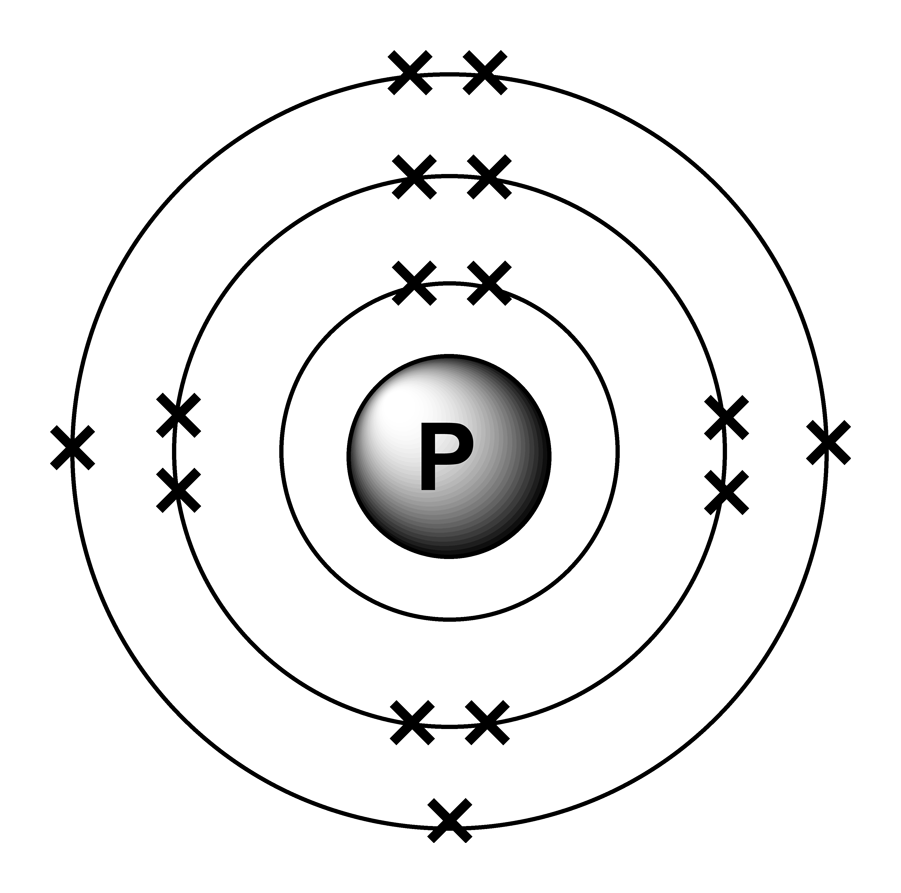
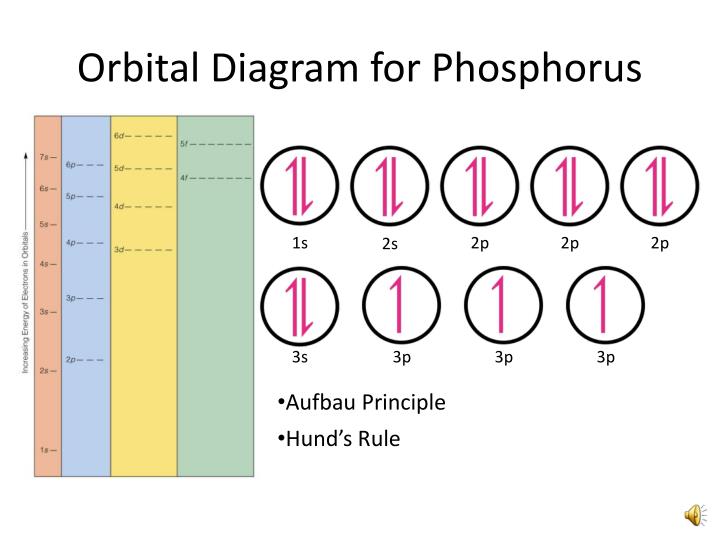
The orbital diagram of phosphorus shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, and the 3p subshell has 3 electrons. How to Write the Orbital Diagram for Phosphorus (P) Watch on Contents Steps Find electrons Write electron configuration

Phosphorus, atomic structure Stock Image C018/3696 Science Photo
To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number.
Electron arrangements
Aufbau Principle: The word 'Aufbau' in German means 'building up'. The Aufbau rule simply gives the order of electrons filling in the orbital of an atom in its ground state. It states that the orbital with the lowest energy level will be filled first before those with high energy levels.

37 draw the orbital diagram for the ion co2+ Diagram Online Source
In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.

P Orbital diagram How to draw orbital diagram of Phosphorus YouTube
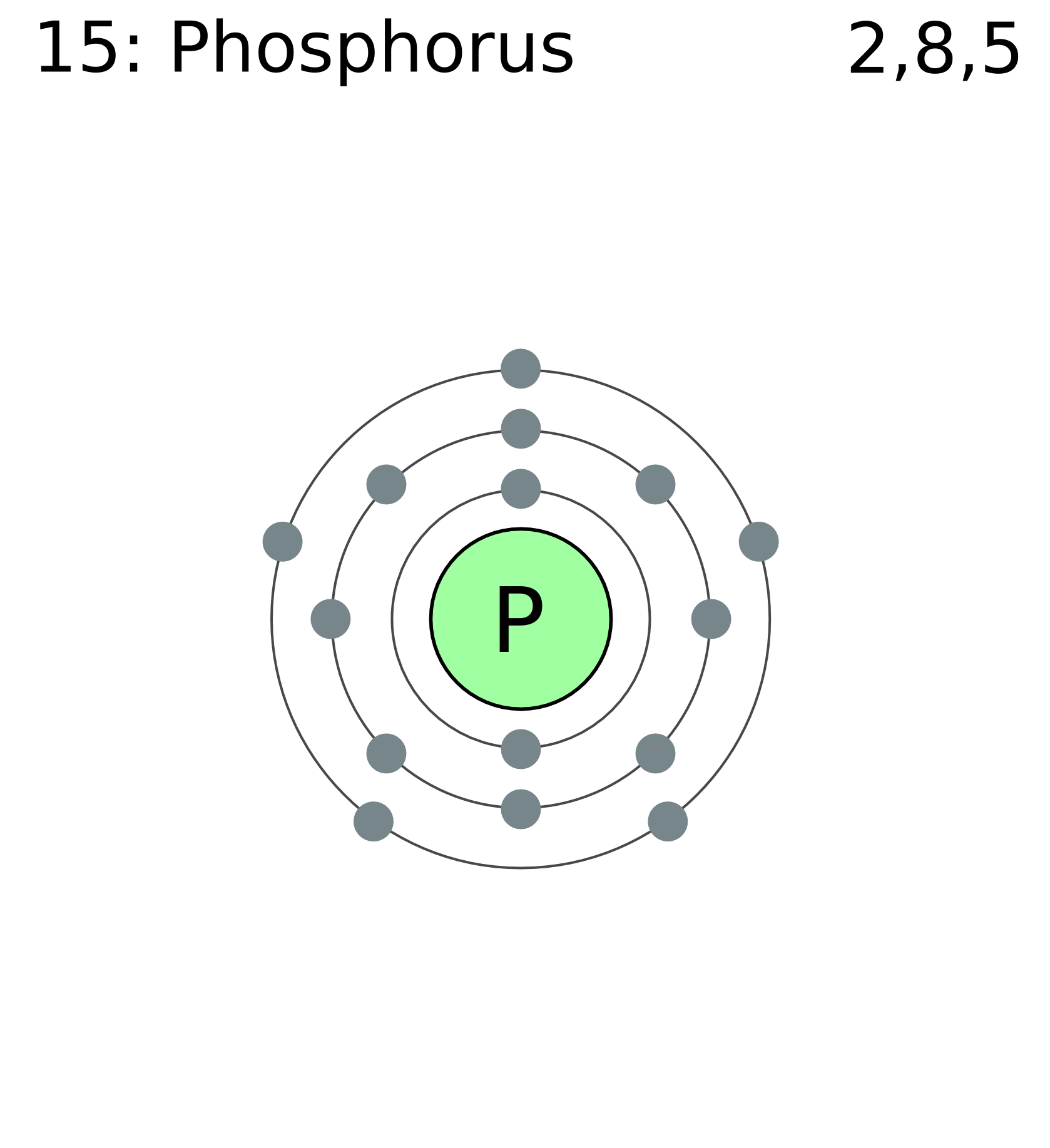
The arrangement of electrons in phosphorus in specific rules in different orbits and orbitals is called the electron configuration of phosphorus. The electron configuration of phosphorus is [ Ne] 3s 2 3p 3 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
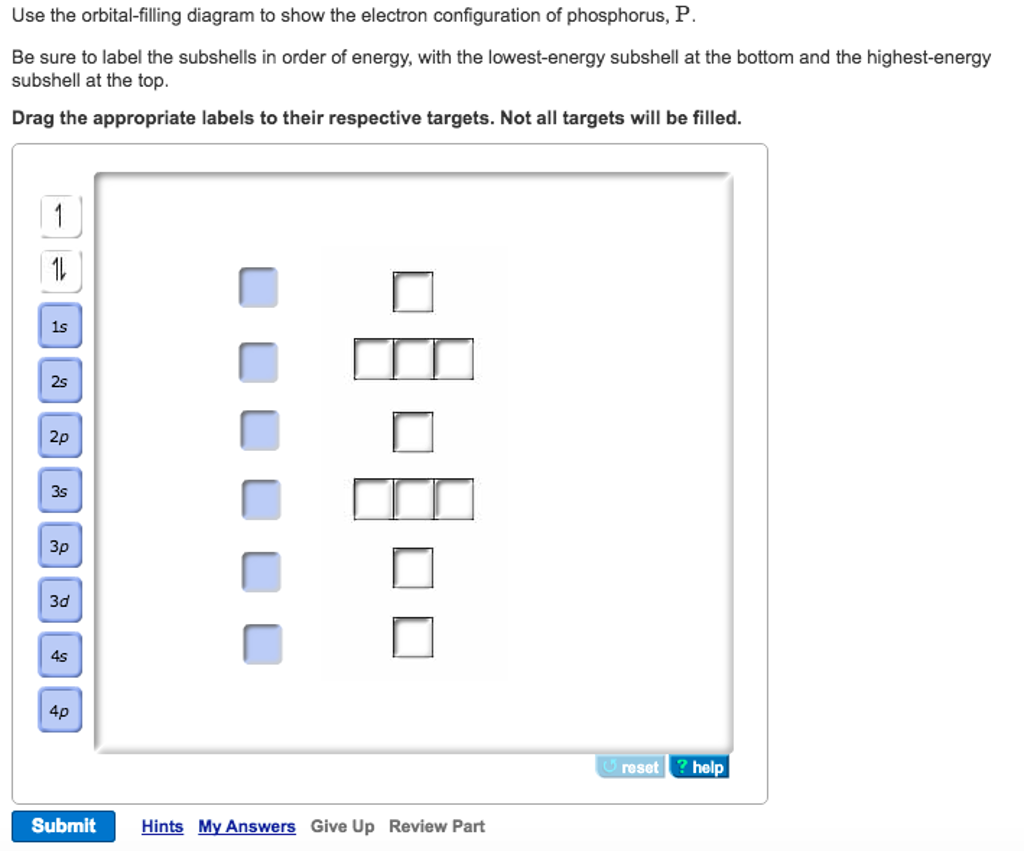
Use The Orbital Filling Diagram To Show The Electron Configuration Of
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
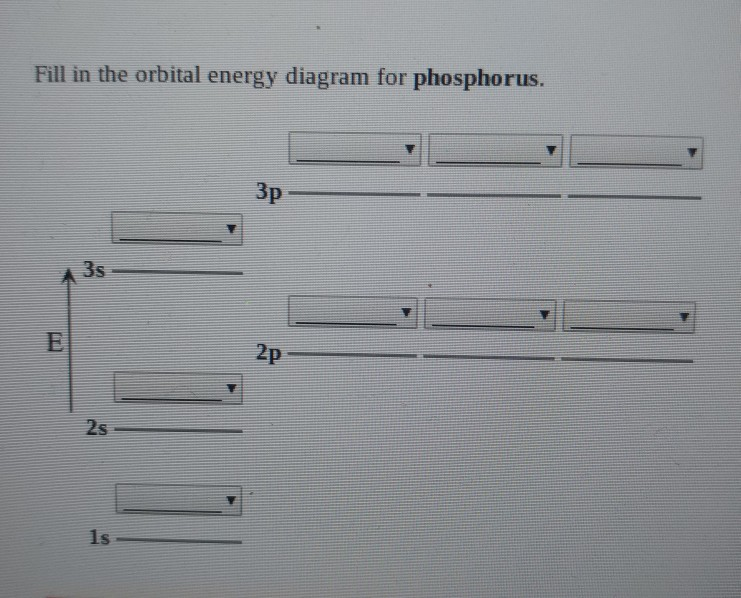
Solved Fill in the orbital energy diagram for phosphorus. 3p
1 2 3 4 5 6 7 8 9 Share 400 views 9 months ago Electron Configuration In this video, we will see what orbital diagrams are, the rules that we follow while determining one for any element and.

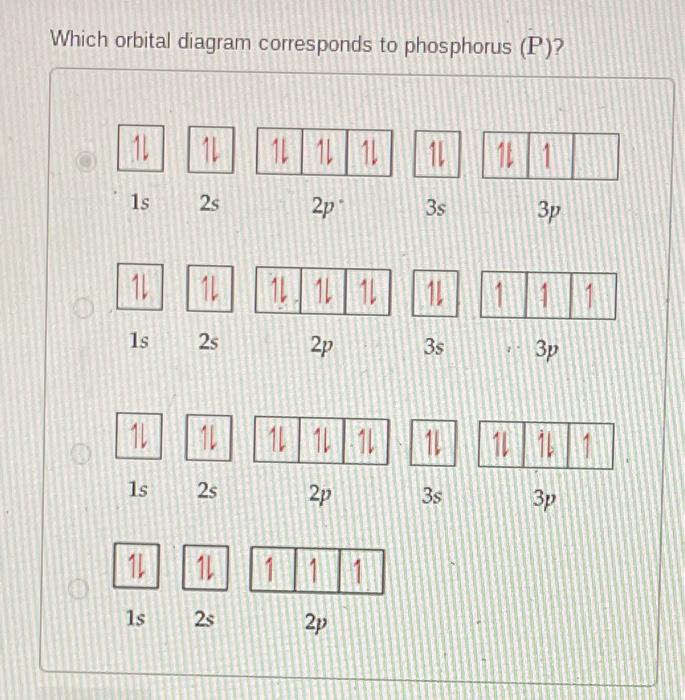
Solved What is the correct orbital diagram for phosphorus?
The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct.

Phosphorus Orbital Diagram
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1. 3 or 5.1. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
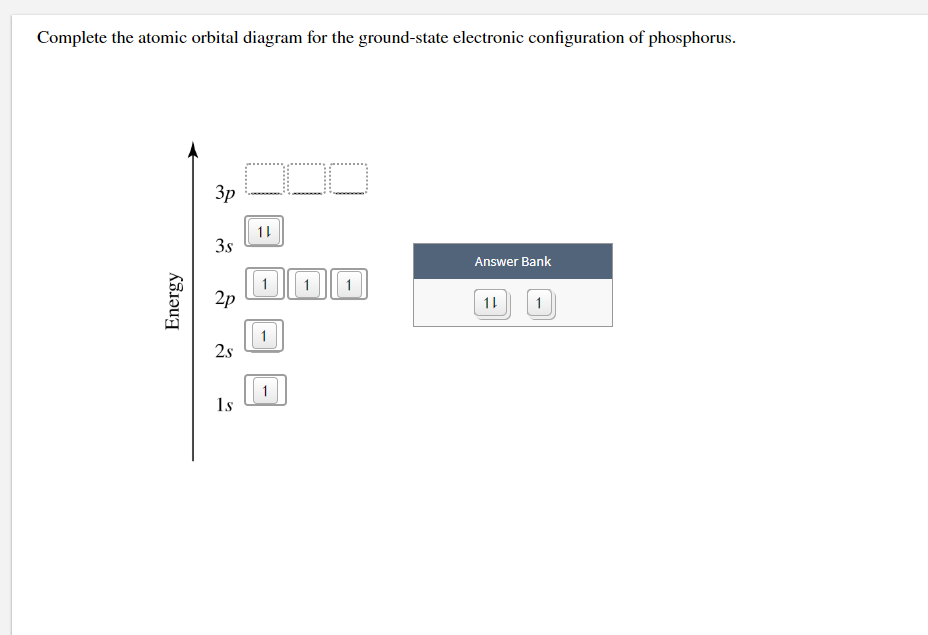
Solved Complete the atomic orbital diagram for the
The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. 1.4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom indicates the number of valence.
PPT Orbital Filling Electron Configurations PowerPoint Presentation
Draw an orbital diagram using the shorthand Nobel Gas Configuration and use it to derive the electron configuration of phosphorus, Z = 15. How many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? Given: atomic number. Asked for: orbital diagram and valence electron configuration for phosphorus